|
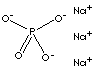
TRISODIUM PHOSPHATE (12 HYDRATE) |
||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 7601-54-9;
96337-98-3 (Anhydrous) 10101-89-0 (Dodecahydrate) |
|
| EINECS NO. | 231-509-8 | |
| FORMULA | Na3PO4·12H2O | |
| MOL WT. | 380.12 |
|
| H.S. CODE | ||
|
TOXICITY |
Oral rat LD50: 7400 mg/kg | |
| SYNONYMS | Trisodium Orthophosphate; | |
| Phosphoric acid, trisodium, 12-hydrate; Sodium Phosphate Tribasic Dodecahydrate; Trisodium phosphate, dodecahydrate; TSP dodecahydrate; Tertiary Sodium phosphate; | ||
| SMILES | P(=O)([O-])([O-])[O-].O.[Na+].[Na+].[Na+].O.O.O.O.O.O.O.O.O.O.O | |
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
White Crystal |
|
| MELTING POINT | 75 C (Decomposes) |
|
| BOILING POINT |
|
|
| SPECIFIC GRAVITY | 1.62 |
|
| SOLUBILITY IN WATER |
Soluble |
|
| pH | 11.5 - 12.2 (1% sol) | |
| VAPOR DENSITY | ||
| AUTOIGNITION |
|
|
| NFPA RATINGS | Health: 3; Flammability: 0; Reactivity: 0 | |
|
REFRACTIVE INDEX |
||
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & EXTERNAL LINKS |
||
|
photographic developers; Removing boiler scale, Softening water; detergent mixture; Wet finishing - pH value adjustment; Aluminium work pieces - Polishing, Burnishing; Clarifying sugar, Fermentation - Nutrient; Complementary feeding stuff for livestock breeding - Mineral enrichment Wikipedia Linking: http://en.wikipedia.org/wiki/Trisodium_phosphate Material Safety Data Sheet: http://www.inchem.org/documents/icsc/icsc/eics1178.htm Google Scholar Search: http://scholar.google.com/ Trisodium phosphate poisoning: http://www.nlm.nih.gov/medlineplus/ency/article/002489.htmTSP is used for washing surfaces prior to painting, especially exterior
surfaces. Liquid bleach is often added to TSP if there is mildew on the
surfaces. The TSP and bleach act in concert to both kill the mildew and remove
its characteristic stains. It may be used on inside surfaces also, but try to
mask all surfaces except the one you want to clean. It can damage many metal and
painted surfaces, and can stain woods. It is not recommended for use on glass,
either, since it will leave a filmy residue. Phosphates Use in Foods: http://www.foodadditives.org/ Latest Trisodium Phosphate Laundry Documents: http://www.vssm.org/ |
||
| SALES SPECIFICATION | ||
|
FOOD GRADE (FCC) |
||
|
APPEARANCE |
White Crystal |
|
| Na3PO4.12H2O |
98.0 - 102.0% |
|
| SULPHATE (as SO4) |
0.5% max |
|
| CHLORIDE (as Cl) |
0.3% max |
|
|
WATER INSOLUBLES |
0.1% max |
|
|
Na2O |
16 - 20.5% |
|
| HEAVY METAL |
10ppm max |
|
| ARSENIC |
3ppmmmax |
|
| LOSS ON IGNITION |
45.0 - 57.0% |
|
|
pH |
11.5 - 12.2 (1% sol) | |
|
TECH GRADE |
||
|
APPEARANCE |
White Crystal |
|
| Na3PO4.12H2O |
98.0% min |
|
| SULPHATE (as SO4) |
0.5% max |
|
| CHLORIDE (as Cl) |
0.3% max |
|
|
WATER INSOLUBLES |
0.1% max |
|
|
Na2O |
16.0 - 19.0% |
|
| TRANSPORTATION | ||
| PACKING | 25kgs , 50kgs in bag | |
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 26/28 | ||